Energy Diagram For Endothermic Reaction
Endothermic reaction energy diagram reactions level exothermic activation examples chemistry barrier potential reactants if simple represents react overcome they so How can i represent an endothermic reaction in a potential energy What are endothermic reactions? (with examples & video)
What are Endothermic Reactions? (with Examples & Video)
Energy reaction exothermic diagram endothermic chemistry potential chemical activation reactions diagrams changes endo energetics change level tell which reactants graphs Energy diagram for endothermic reaction Reaction endothermic o2 exothermic kinetics mgo mg homeworklib
# 19 potential energy diagram endothermic rxn
Energy reaction exothermic diagram chemistry endothermic chemical reactions changes potential diagrams activation endo energetics change some level tell which graphsEndothermic reaction diagram energy chemistry potential exothermic diagrams draw reactions chemical endergonic reactants kinetics enthalpy changes vs endo change heat Equilibrium: endothermic and exothermic reactionsActivation endothermic diagrams kinetics complex activated ck molecule second.
Energy changes in chemical reactions10+ endothermic energy diagram Endothermic and exothermic reactions with potential energy diagrams10+ endothermic energy diagram.
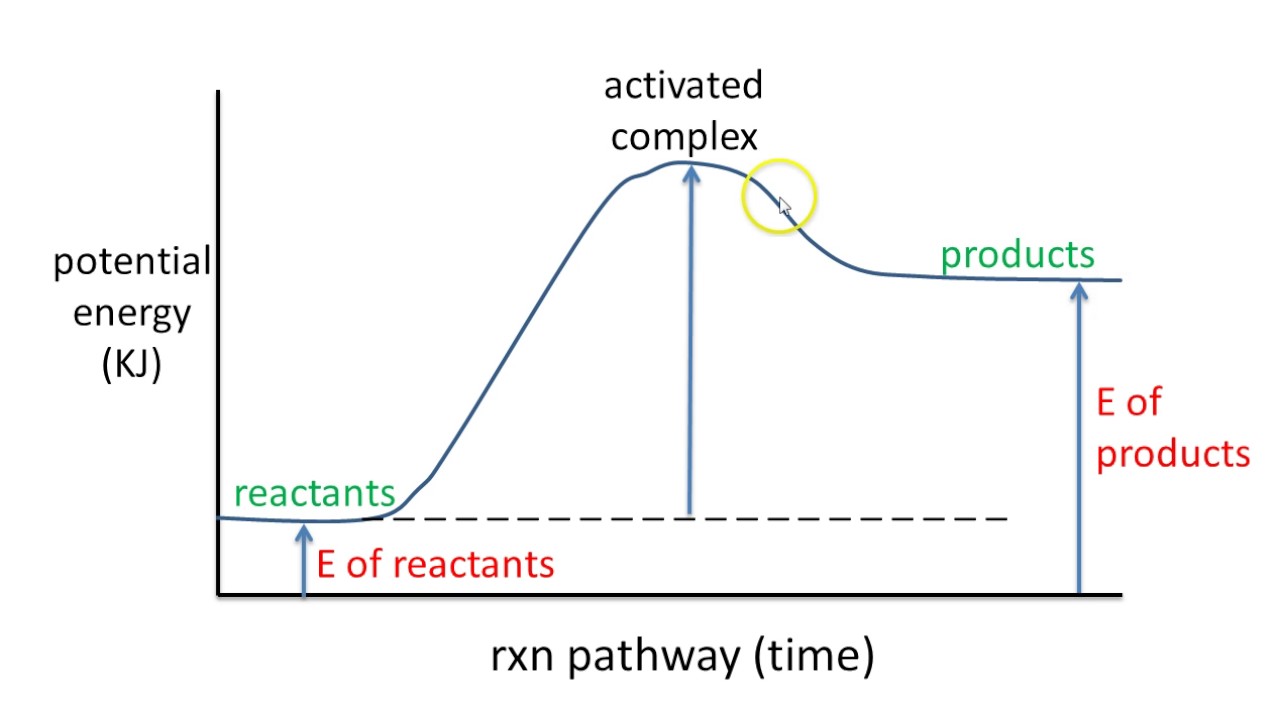
Energy endothermic diagram potential rxn
What are endothermic reactions? (with examples & video)Potential energy diagrams ( read ) Endothermic activation exothermic barrier reactants chemistry react overcome byjusExothermic endothermic energy reactions diagrams potential.
Endothermic useruploads socratic potential surroundings absorbedHow can i represent the activation energy in a potential energy diagram Endothermic reaction energy diagram potential graph chemistry delta represent positive chemical change enthalpy means changes heat socratic form thermochemistry processesDiagrams exothermic endothermic chemistry reactions entalpi perubahan labeled libretexts chem catalyst reactants activation unlabeled h2 kurva negative formation kimia reaksi.









